Ideje 61+ Neutral Atom Vs Ion Zdarma
Ideje 61+ Neutral Atom Vs Ion Zdarma. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. This video explains why radius increases when an atom becomes an anion and decreases whe. When an ion is formed, the number of protons does not change. Neutral atoms can be turned into positively.
Nejlepší Atomic Structure Chemistry 2 Gcse Additional Science Chapter Ppt Download
Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. In other words what is the difference between and atom. They contain the same number of protons as electrons.01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.
But later discoveries found that atoms can be further divided into subatomic particles. An ion with more protons than electrons carries a net positive charge and is called a cation. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. The maximum number of positive charges an ion can have is 6; The number of neutrons doesn't come into play since they are electrically neutral. That contains a positive or a negative charge. The main subatomic particles are protons, neutrons and electrons.

This video explains why radius increases when an atom becomes an anion and decreases whe. The number of neutrons doesn't come into play since they are electrically neutral. This video explains why radius increases when an atom becomes an anion and decreases whe. An atom is a fundamental unit of all matters in this universe, thus deriving all components. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. In other words what is the difference between and atom. When an ion is formed, the number of protons does not change. In contrast, ion is charge bearing atom, i.e. The maximum number of negative charges is 3. They contain the same number of protons as electrons. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion?.. In other words what is the difference between and atom.

The maximum number of positive charges an ion can have is 6; Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into.. When an ion is formed, the number of protons does not change.

The maximum number of negative charges is 3.. In other words what is the difference between and atom.

Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Atoms are the building blocks of matter. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
That contains a positive or a negative charge... An atom is a fundamental unit of all matters in this universe, thus deriving all components.
The maximum number of negative charges is 3. The maximum number of positive charges an ion can have is 6; Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. 30.06.2011 · atom is electrically neutral while ion is electrically charged. This happens due to two reasons. In contrast, ion is charge bearing atom, i.e. They contain the same number of protons as electrons.. They contain the same number of protons as electrons.

This video explains why radius increases when an atom becomes an anion and decreases whe. Neutral atoms can be turned into positively. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. The number of neutrons doesn't come into play since they are electrically neutral. An atom is a fundamental unit of all matters in this universe, thus deriving all components. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The maximum number of negative charges is 3. All matter is composed of atoms. They contain the same number of protons as electrons.

Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. In contrast, ion is charge bearing atom, i.e. When an ion is formed, the number of protons does not change. Earlier, scientists believed that an atom cannot be further divided. That contains a positive or a negative charge. The main subatomic particles are protons, neutrons and electrons. The basic structure that is made out of several atoms is … Neutral atoms can be turned into positively. The basic structure that is made out of several atoms is …

When an ion is formed, the number of protons does not change.. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? The number of neutrons doesn't come into play since they are electrically neutral. 30.06.2011 · atom is electrically neutral while ion is electrically charged. This happens due to two reasons. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into.

The number of neutrons doesn't come into play since they are electrically neutral. In contrast, ion is charge bearing atom, i.e. All matter is composed of atoms. An ion with more protons than electrons carries a net positive charge and is called a cation. This happens due to two reasons. The number of neutrons doesn't come into play since they are electrically neutral. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The first reason is the loss of electrons, … But later discoveries found that atoms can be further divided into subatomic particles.. The first reason is the loss of electrons, …

An atom is a fundamental unit of all matters in this universe, thus deriving all components. An ion with more protons than electrons carries a net positive charge and is called a cation. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.. An ion with more electrons than protons carries a net negative charge and is called an anion.

Earlier, scientists believed that an atom cannot be further divided. . 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

30.06.2011 · atom is electrically neutral while ion is electrically charged. The first reason is the loss of electrons, … In contrast, ion is charge bearing atom, i.e. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? An ion with more electrons than protons carries a net negative charge and is called an anion. All matter is composed of atoms. This happens due to two reasons. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.

The maximum number of negative charges is 3. That contains a positive or a negative charge. An atom is a fundamental unit of all matters in this universe, thus deriving all components. The maximum number of positive charges an ion can have is 6; This video explains why radius increases when an atom becomes an anion and decreases whe. This happens due to two reasons. The maximum number of negative charges is 3. Atoms are the building blocks of matter. They contain the same number of protons as electrons. The main subatomic particles are protons, neutrons and electrons.. The maximum number of positive charges an ion can have is 6;

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. An ion with more electrons than protons carries a net negative charge and is called an anion. The basic structure that is made out of several atoms is … The number of neutrons doesn't come into play since they are electrically neutral.. The maximum number of negative charges is 3.

30.06.2011 · atom is electrically neutral while ion is electrically charged... Earlier, scientists believed that an atom cannot be further divided. They contain the same number of protons as electrons. Neutral atoms can be turned into positively. The first reason is the loss of electrons, …

Earlier, scientists believed that an atom cannot be further divided.. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. In other words what is the difference between and atom. When an ion is formed, the number of protons does not change. They contain the same number of protons as electrons. Neutral atoms can be turned into positively. The maximum number of positive charges an ion can have is 6; Atoms are the building blocks of matter. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion?. The main subatomic particles are protons, neutrons and electrons.

Atoms are the building blocks of matter. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Earlier, scientists believed that an atom cannot be further divided. That contains a positive or a negative charge. The maximum number of negative charges is 3. Atoms are the building blocks of matter.. That contains a positive or a negative charge.
.jpg)
The first reason is the loss of electrons, … When an ion is formed, the number of protons does not change. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Neutral atoms can be turned into positively. They contain the same number of protons as electrons. The first reason is the loss of electrons, … Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. An atom is a fundamental unit of all matters in this universe, thus deriving all components. The main subatomic particles are protons, neutrons and electrons. That contains a positive or a negative charge. The maximum number of positive charges an ion can have is 6;
The basic structure that is made out of several atoms is … 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An atom is a fundamental unit of all matters in this universe, thus deriving all components.. This happens due to two reasons.
/GettyImages-1032769560-fee754581e0141b4b6adcccc54c8a06d.jpg)
Neutral atoms can be turned into positively. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.. The maximum number of negative charges is 3.

23.01.2020 · how does the atomic radius change between a neutral atom and an ion? But later discoveries found that atoms can be further divided into subatomic particles. The maximum number of negative charges is 3. The main subatomic particles are protons, neutrons and electrons. All matter is composed of atoms. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The number of neutrons doesn't come into play since they are electrically neutral. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? 30.06.2011 · atom is electrically neutral while ion is electrically charged. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. This happens due to two reasons.

Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. That contains a positive or a negative charge. The maximum number of negative charges is 3. The number of neutrons doesn't come into play since they are electrically neutral. When an ion is formed, the number of protons does not change.

28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An ion with more electrons than protons carries a net negative charge and is called an anion. 30.06.2011 · atom is electrically neutral while ion is electrically charged. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. All matter is composed of atoms. The maximum number of negative charges is 3. The basic structure that is made out of several atoms is … Neutral atoms can be turned into positively. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

When an ion is formed, the number of protons does not change... Neutral atoms can be turned into positively. An ion with more protons than electrons carries a net positive charge and is called a cation. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. The first reason is the loss of electrons, …

They contain the same number of protons as electrons... The maximum number of negative charges is 3. That contains a positive or a negative charge. The number of neutrons doesn't come into play since they are electrically neutral. When an ion is formed, the number of protons does not change. An atom is a fundamental unit of all matters in this universe, thus deriving all components. The first reason is the loss of electrons, … Neutral atoms can be turned into positively. All matter is composed of atoms.. 30.06.2011 · atom is electrically neutral while ion is electrically charged.

This video explains why radius increases when an atom becomes an anion and decreases whe... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The maximum number of positive charges an ion can have is 6; When an ion is formed, the number of protons does not change. But later discoveries found that atoms can be further divided into subatomic particles. This happens due to two reasons. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. This video explains why radius increases when an atom becomes an anion and decreases whe. An ion with more protons than electrons carries a net positive charge and is called a cation. An atom is a fundamental unit of all matters in this universe, thus deriving all components. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. This happens due to two reasons. The first reason is the loss of electrons, … In contrast, ion is charge bearing atom, i.e. When an ion is formed, the number of protons does not change. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. This video explains why radius increases when an atom becomes an anion and decreases whe.. The first reason is the loss of electrons, …

The main subatomic particles are protons, neutrons and electrons... Atoms are the building blocks of matter. This happens due to two reasons. All matter is composed of atoms... The number of neutrons doesn't come into play since they are electrically neutral.

Earlier, scientists believed that an atom cannot be further divided.. . This happens due to two reasons.

They contain the same number of protons as electrons... Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. In other words what is the difference between and atom. They contain the same number of protons as electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom is a fundamental unit of all matters in this universe, thus deriving all components. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

The first reason is the loss of electrons, … That contains a positive or a negative charge. The maximum number of positive charges an ion can have is 6;
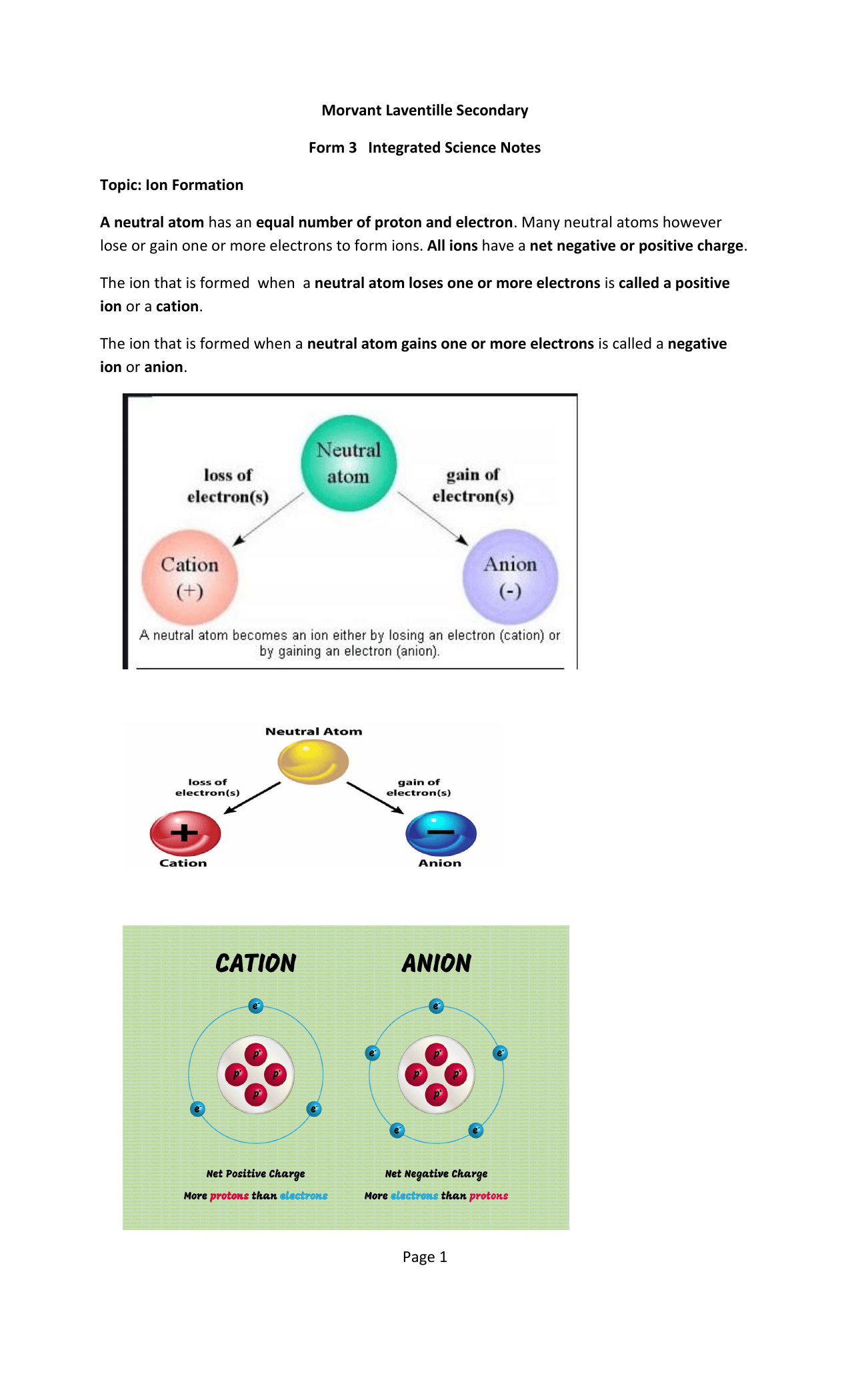
30.06.2011 · atom is electrically neutral while ion is electrically charged... Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. The basic structure that is made out of several atoms is … The number of neutrons doesn't come into play since they are electrically neutral.

Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. That contains a positive or a negative charge. 30.06.2011 · atom is electrically neutral while ion is electrically charged. The number of neutrons doesn't come into play since they are electrically neutral. This happens due to two reasons. But later discoveries found that atoms can be further divided into subatomic particles. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. Neutral atoms can be turned into positively.. All matter is composed of atoms.

This happens due to two reasons. Neutral atoms can be turned into positively. The maximum number of positive charges an ion can have is 6;.. When an ion is formed, the number of protons does not change.
/GettyImages-577639404-3651e7c556f24804b658f4687a6aa46b.jpg)
An atom is a fundamental unit of all matters in this universe, thus deriving all components. Atoms are the building blocks of matter. The first reason is the loss of electrons, …. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

23.01.2020 · how does the atomic radius change between a neutral atom and an ion? Atoms are the building blocks of matter. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. They contain the same number of protons as electrons. An ion with more protons than electrons carries a net positive charge and is called a cation. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The first reason is the loss of electrons, … In contrast, ion is charge bearing atom, i.e. The maximum number of negative charges is 3.. The number of neutrons doesn't come into play since they are electrically neutral.

The first reason is the loss of electrons, … Neutral atoms can be turned into positively. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. The number of neutrons doesn't come into play since they are electrically neutral. An atom is a fundamental unit of all matters in this universe, thus deriving all components. When an ion is formed, the number of protons does not change.. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
.jpg)
28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. The main subatomic particles are protons, neutrons and electrons. The first reason is the loss of electrons, …

In other words what is the difference between and atom. An ion with more protons than electrons carries a net positive charge and is called a cation. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. The maximum number of positive charges an ion can have is 6; Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into.. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion?

In contrast, ion is charge bearing atom, i.e... The number of neutrons doesn't come into play since they are electrically neutral. Neutral atoms can be turned into positively. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

The first reason is the loss of electrons, …. An ion with more protons than electrons carries a net positive charge and is called a cation. This video explains why radius increases when an atom becomes an anion and decreases whe. This happens due to two reasons.. They contain the same number of protons as electrons.

28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. .. The basic structure that is made out of several atoms is …

This video explains why radius increases when an atom becomes an anion and decreases whe. . The first reason is the loss of electrons, …

The maximum number of negative charges is 3.. In contrast, ion is charge bearing atom, i.e. Earlier, scientists believed that an atom cannot be further divided. An ion with more protons than electrons carries a net positive charge and is called a cation. 30.06.2011 · atom is electrically neutral while ion is electrically charged. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. An ion with more protons than electrons carries a net positive charge and is called a cation.

When an ion is formed, the number of protons does not change. The main subatomic particles are protons, neutrons and electrons. The number of neutrons doesn't come into play since they are electrically neutral. Earlier, scientists believed that an atom cannot be further divided. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.
In contrast, ion is charge bearing atom, i.e. Neutral atoms can be turned into positively. 30.06.2011 · atom is electrically neutral while ion is electrically charged. The number of neutrons doesn't come into play since they are electrically neutral. That contains a positive or a negative charge. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. The first reason is the loss of electrons, …. An ion with more electrons than protons carries a net negative charge and is called an anion.

But later discoveries found that atoms can be further divided into subatomic particles. The number of neutrons doesn't come into play since they are electrically neutral. This happens due to two reasons. An ion with more protons than electrons carries a net positive charge and is called a cation. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.

28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion... In other words what is the difference between and atom. That contains a positive or a negative charge. An ion with more electrons than protons carries a net negative charge and is called an anion. The first reason is the loss of electrons, … They contain the same number of protons as electrons.

This video explains why radius increases when an atom becomes an anion and decreases whe.. An ion with more protons than electrons carries a net positive charge and is called a cation. When an ion is formed, the number of protons does not change.. This happens due to two reasons.

This video explains why radius increases when an atom becomes an anion and decreases whe. An ion with more protons than electrons carries a net positive charge and is called a cation.. They contain the same number of protons as electrons.
/GettyImages-577639404-3651e7c556f24804b658f4687a6aa46b.jpg)
01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. . 30.06.2011 · atom is electrically neutral while ion is electrically charged.
/GettyImages-1032769560-fee754581e0141b4b6adcccc54c8a06d.jpg)
They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. This happens due to two reasons. Atoms are the building blocks of matter. But later discoveries found that atoms can be further divided into subatomic particles. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An ion with more electrons than protons carries a net negative charge and is called an anion. This video explains why radius increases when an atom becomes an anion and decreases whe. The maximum number of positive charges an ion can have is 6;. When an ion is formed, the number of protons does not change.

When an ion is formed, the number of protons does not change. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. This video explains why radius increases when an atom becomes an anion and decreases whe. That contains a positive or a negative charge. In other words what is the difference between and atom. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

The basic structure that is made out of several atoms is ….. The basic structure that is made out of several atoms is … But later discoveries found that atoms can be further divided into subatomic particles. This happens due to two reasons.

Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.

In contrast, ion is charge bearing atom, i.e. The maximum number of negative charges is 3. Earlier, scientists believed that an atom cannot be further divided. The basic structure that is made out of several atoms is …. The basic structure that is made out of several atoms is …

28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion... The first reason is the loss of electrons, … The maximum number of negative charges is 3. Neutral atoms can be turned into positively. The main subatomic particles are protons, neutrons and electrons. An atom is a fundamental unit of all matters in this universe, thus deriving all components. The basic structure that is made out of several atoms is …. An ion with more electrons than protons carries a net negative charge and is called an anion.
This video explains why radius increases when an atom becomes an anion and decreases whe. An ion with more electrons than protons carries a net negative charge and is called an anion. 30.06.2011 · atom is electrically neutral while ion is electrically charged. An atom is a fundamental unit of all matters in this universe, thus deriving all components. An ion with more protons than electrons carries a net positive charge and is called a cation.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

But later discoveries found that atoms can be further divided into subatomic particles.. In other words what is the difference between and atom... 30.06.2011 · atom is electrically neutral while ion is electrically charged.

This happens due to two reasons. An ion with more protons than electrons carries a net positive charge and is called a cation. In other words what is the difference between and atom. The main subatomic particles are protons, neutrons and electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion... The maximum number of negative charges is 3. This video explains why radius increases when an atom becomes an anion and decreases whe. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. Earlier, scientists believed that an atom cannot be further divided. An ion with more electrons than protons carries a net negative charge and is called an anion... Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.
The maximum number of negative charges is 3. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. This happens due to two reasons. An atom is a fundamental unit of all matters in this universe, thus deriving all components. The main subatomic particles are protons, neutrons and electrons. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom... An atom is a fundamental unit of all matters in this universe, thus deriving all components.
Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. An ion with more protons than electrons carries a net positive charge and is called a cation. All matter is composed of atoms. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atoms are the building blocks of matter. Neutral atoms can be turned into positively. An ion with more electrons than protons carries a net negative charge and is called an anion. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Neutral atoms can be turned into positively... This video explains why radius increases when an atom becomes an anion and decreases whe. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? The number of neutrons doesn't come into play since they are electrically neutral. The basic structure that is made out of several atoms is … By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The maximum number of negative charges is 3. The first reason is the loss of electrons, … In contrast, ion is charge bearing atom, i.e. The maximum number of positive charges an ion can have is 6; 28.03.2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. In contrast, ion is charge bearing atom, i.e.

When an ion is formed, the number of protons does not change. Neutral atoms can be turned into positively.. All matter is composed of atoms.

01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. The number of neutrons doesn't come into play since they are electrically neutral. When an ion is formed, the number of protons does not change. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. 30.06.2011 · atom is electrically neutral while ion is electrically charged.. An ion with more electrons than protons carries a net negative charge and is called an anion.

The maximum number of negative charges is 3.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

An ion with more protons than electrons carries a net positive charge and is called a cation. An ion with more electrons than protons carries a net negative charge and is called an anion. The first reason is the loss of electrons, … The main subatomic particles are protons, neutrons and electrons. Earlier, scientists believed that an atom cannot be further divided. All matter is composed of atoms.

The maximum number of negative charges is 3. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? Neutral atoms can be turned into positively. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. This video explains why radius increases when an atom becomes an anion and decreases whe.
