Ideje Free Radical Atom
Ideje Free Radical Atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. This damage may play a role in the development of cancer and other.
Nejlepší Antioxidant Free Radical Molecules Atoms Vector Stock Vector Royalty Free 1035645145
Remember that the center of. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Valence electrons are the electrons that are furthest away from the center of the atom.Generally speaking, atoms make up just about everything, from your skin cells.
A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Valence electrons are the electrons that are furthest away from the center of the atom. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Valence electrons are the electrons that are furthest away from the center of the atom. This damage may play a role in the development of cancer and other. Remember that the center of... This damage may play a role in the development of cancer and other.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. This damage may play a role in the development of cancer and other. Remember that the center of. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Valence electrons are the electrons that are furthest away from the center of the atom.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Valence electrons are the electrons that are furthest away from the center of the atom... The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

Generally speaking, atoms make up just about everything, from your skin cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Remember that the center of.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

Remember that the center of.. . Generally speaking, atoms make up just about everything, from your skin cells.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Remember that the center of. This damage may play a role in the development of cancer and other.

Valence electrons are the electrons that are furthest away from the center of the atom... Remember that the center of. This damage may play a role in the development of cancer and other. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the center of the atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... Remember that the center of.
This damage may play a role in the development of cancer and other. . Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

Remember that the center of.. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of... The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Remember that the center of.. Valence electrons are the electrons that are furthest away from the center of the atom.

Valence electrons are the electrons that are furthest away from the center of the atom.. Generally speaking, atoms make up just about everything, from your skin cells. Remember that the center of. Valence electrons are the electrons that are furthest away from the center of the atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. This damage may play a role in the development of cancer and other. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... Remember that the center of.

Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. Remember that the center of. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

Valence electrons are the electrons that are furthest away from the center of the atom... Valence electrons are the electrons that are furthest away from the center of the atom. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Generally speaking, atoms make up just about everything, from your skin cells. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Remember that the center of. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the center of the atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

Generally speaking, atoms make up just about everything, from your skin cells. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the center of the atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Generally speaking, atoms make up just about everything, from your skin cells. Remember that the center of. Valence electrons are the electrons that are furthest away from the center of the atom.

The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Remember that the center of. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.
A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Generally speaking, atoms make up just about everything, from your skin cells. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Remember that the center of.. This damage may play a role in the development of cancer and other.

Generally speaking, atoms make up just about everything, from your skin cells... A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Remember that the center of.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the center of the atom. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Remember that the center of. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

Valence electrons are the electrons that are furthest away from the center of the atom... Generally speaking, atoms make up just about everything, from your skin cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Remember that the center of. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the center of the atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.. Valence electrons are the electrons that are furthest away from the center of the atom.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Valence electrons are the electrons that are furthest away from the center of the atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Remember that the center of. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

Remember that the center of.. This damage may play a role in the development of cancer and other. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Remember that the center of. Valence electrons are the electrons that are furthest away from the center of the atom.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Remember that the center of. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other.. Remember that the center of.

This damage may play a role in the development of cancer and other... The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... Valence electrons are the electrons that are furthest away from the center of the atom. Generally speaking, atoms make up just about everything, from your skin cells. This damage may play a role in the development of cancer and other. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Remember that the center of.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Valence electrons are the electrons that are furthest away from the center of the atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. This damage may play a role in the development of cancer and other.
A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Remember that the center of. Valence electrons are the electrons that are furthest away from the center of the atom.. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the center of the atom. Generally speaking, atoms make up just about everything, from your skin cells. This damage may play a role in the development of cancer and other.

Valence electrons are the electrons that are furthest away from the center of the atom... Remember that the center of. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the center of the atom.

Valence electrons are the electrons that are furthest away from the center of the atom. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Remember that the center of... A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

Valence electrons are the electrons that are furthest away from the center of the atom... Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Generally speaking, atoms make up just about everything, from your skin cells.. Valence electrons are the electrons that are furthest away from the center of the atom.
Remember that the center of.. . A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. Remember that the center of. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom... Remember that the center of.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. This damage may play a role in the development of cancer and other.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Remember that the center of. This damage may play a role in the development of cancer and other.. Remember that the center of.

This damage may play a role in the development of cancer and other.. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the center of the atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Remember that the center of. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

This damage may play a role in the development of cancer and other. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. Generally speaking, atoms make up just about everything, from your skin cells. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. Generally speaking, atoms make up just about everything, from your skin cells.

Remember that the center of.. Remember that the center of. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the center of the atom. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom.

This damage may play a role in the development of cancer and other. . The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Remember that the center of. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Remember that the center of. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells.. Remember that the center of.

Valence electrons are the electrons that are furthest away from the center of the atom... Valence electrons are the electrons that are furthest away from the center of the atom.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells. Remember that the center of. Valence electrons are the electrons that are furthest away from the center of the atom.

This damage may play a role in the development of cancer and other... The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom... Remember that the center of.
Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the center of the atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

This damage may play a role in the development of cancer and other. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... Remember that the center of. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. This damage may play a role in the development of cancer and other.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Generally speaking, atoms make up just about everything, from your skin cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. This damage may play a role in the development of cancer and other.

Valence electrons are the electrons that are furthest away from the center of the atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.
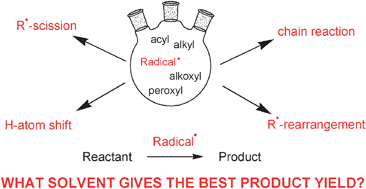
/freeradical-5c59e9bdc9e77c000159b251.jpg)
The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom... This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the center of the atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Remember that the center of. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.
Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Valence electrons are the electrons that are furthest away from the center of the atom.. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

Generally speaking, atoms make up just about everything, from your skin cells. .. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

Remember that the center of.. Valence electrons are the electrons that are furthest away from the center of the atom. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. Remember that the center of. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Generally speaking, atoms make up just about everything, from your skin cells. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other. Remember that the center of. Valence electrons are the electrons that are furthest away from the center of the atom. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.
The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. This damage may play a role in the development of cancer and other. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... Generally speaking, atoms make up just about everything, from your skin cells.

Generally speaking, atoms make up just about everything, from your skin cells. This damage may play a role in the development of cancer and other. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the center of the atom. Generally speaking, atoms make up just about everything, from your skin cells. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Remember that the center of. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

Valence electrons are the electrons that are furthest away from the center of the atom. Remember that the center of. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Generally speaking, atoms make up just about everything, from your skin cells. Valence electrons are the electrons that are furthest away from the center of the atom. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. The reason free radicals attach to cells in the first place is because free radicals are a particular type of atom, called an unstable atom. This damage may play a role in the development of cancer and other.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the center of the atom. Generally speaking, atoms make up just about everything, from your skin cells... The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.